Full Compliance and Traceability
With Zeria’s deep understanding of Chondroitin and its potential as a pharmaceutical product, the company consistently explores innovative business solutions to further its expansion in this field. Placing top priority in securing the highest-possible quality of its raw materials, Zeria acquired ZPD A/S (formerly known as Biofac Esbjerg A/S) in 2010, a manufacturer and developer of glycosaminoglycan Chondroitin Sulfate sodium, which now serves as an independent subsidiary under the Zeria group, making it possible for Zeria to achieve stable procurement of high-quality Chondroitin materials in its production of Chondroitin pharmaceutical products.
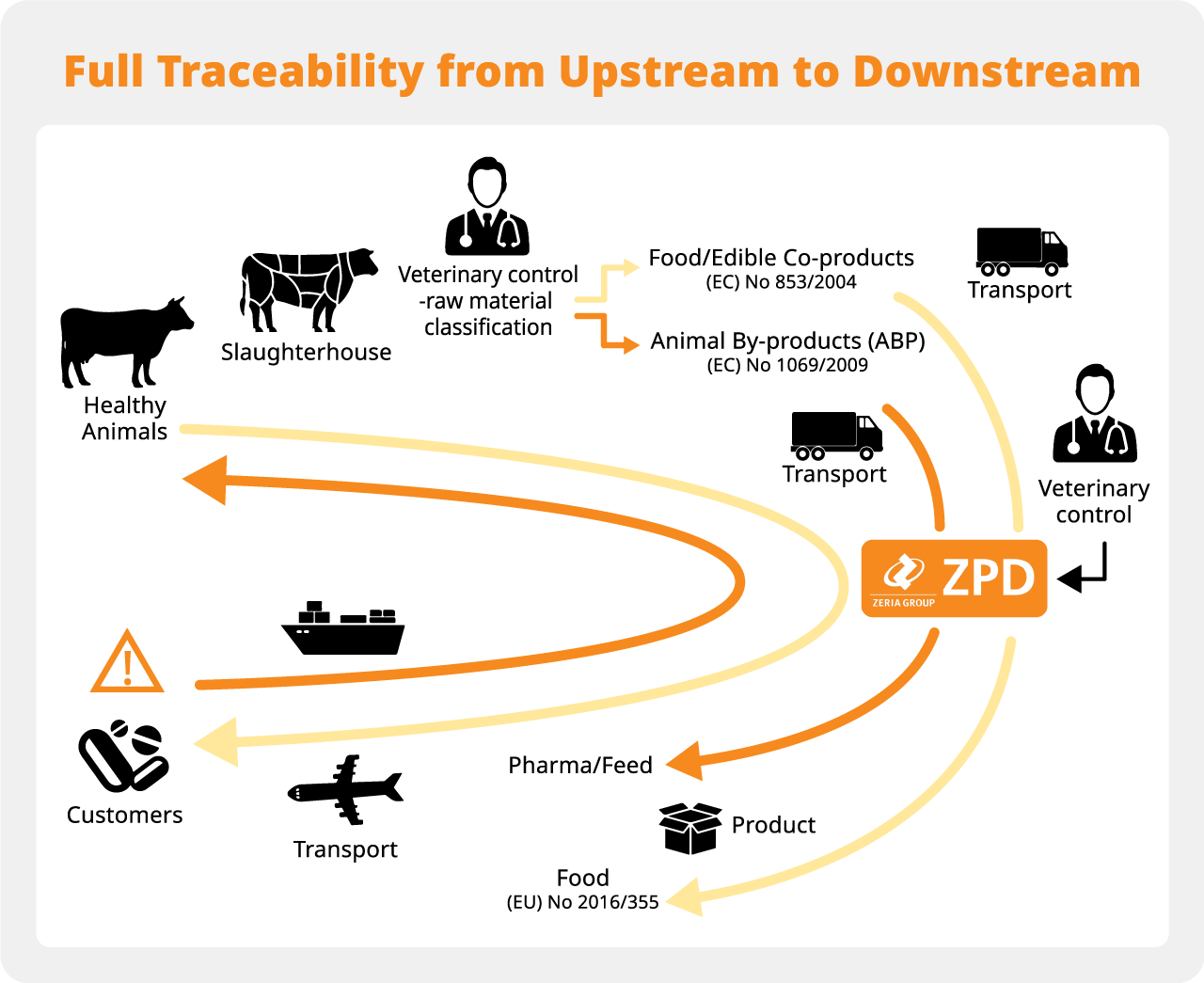
The Denmark-based production facility adheres to stringent regulations stipulated by the European Union, executing rigorous quality control measures throughout its entire manufacturing process from upstream to downstream, enabling full traceability to ensure the efficacy and safety of its products.

Accreditations obtained by ZPD A/S
Drug Manufacturing License (Danish Medicines Agency)
GMP certification for Chondroitin Sulfate Sodium
HACCP Principles
Food Authorisation (Danish Veterinary and Food Administration)
Approved for handling Cat. III by Danish Veterinary and Food Administration, according to EC No. 1069/2009
Registration as a Feed Manufacturer - regulation (EC) No. 183/2005
Authorised according to EU regulation 852/2004 + 853/2004 and its amendment EU 2016/355
Accreditation Certificate of Foreign Drug Manufacturer (AG20400038) (Japan)
Registered with the US FDA pursuant to the Federal Food Drug and Cosmetic Act
GMP and ISO-Certified Production Facilities in Japan
In accordance with Zeria’s spirit of manufacturing, the company continuously strives to further improve quality and reliability through an integrated system that encompasses functions ranging from development to production and distribution.
Zeria has built an optimal production structure underpinned by its 4 domestic and 3 overseas production bases, through which it makes its utmost efforts to manufacture high quality and highly reliable products.
The Tsukuba Plant, which produces a wide range of products including Zeria's Chondroitin product range, has obtained certification for Good Manufacturing Practice (GMP) for the category of dietary supplements, as well as ISO 14001, the international standard for environmental management systems.

